What cause fluctuations in pH?
The pH (potential hydrogen) level of any material or substance is defined by the acidity or alkalinity of that substance and is represented on a scale of 0 to 14; 7pH being neutral, 0pH acidic and 14pH alkaline. This factor can affect many parameters in nearly all types of process or production.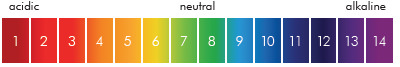
So you set the pH of your hydroponic reservoir to 5.5 only to return 2 days later to find the level has risen to a pH of 6.0! You are wondering why the pH won’t stay where you set it?
The nutrients we add to our reservoir create the fluctuations in pH. When the plant consumes the nutrients, the natural reaction is a rise in pH. This means it is normal for the reservoir to have a natural pH up-swing. It is much more desirable than the pH dropping, which would indicate growers have too much nutrient in your solution.
pH goes up, EC goes down = Plants are feeding. Potentially raise nutrient levels.
pH goes down, EC goes up = Plants are putting nutrient into the water rather than taking them out. Nutrient levels are too high.
pH stays stable, EC levels stay stable = Equilibrium. Plants are taking equal parts nutrients and water. Maximum growth is occurring.
It is best to error on the side of caution and slightly underfeed the plants. This will encourage the natural pH swing from 5.5-6.2. Once the solution reaches the 6.2 mark it should be brought back down to 5.5 pH and allowed to slowly drift up again. The reason this is ideal is that the plants are able to take up specific nutrients more efficiently at different pH levels. By going up and down through the proper pH range the plant has good access to all nutrients required for optimal growth.
Having the ability to monitor and adjust nutrient and pH levels in this fashion are one of the main benefits to an active or recirculating hydroponic system.